Abstract: A new type of copper/nickel/chromium composite coating on plastic parts provided by an auto parts manufacturer is used to evaluate the corrosion resistance of the new trivalent chromium coating by copper accelerated acetic acid-salt fog corrosion and Russian mud corrosion. The results show that compared with the traditional hexavalent chromium system coating, the iron element and the compactness of the coating in the trivalent chromium system coating are the main factors causing the corrosion resistance of the coating to decrease slightly, but the new trivalent chromium system coating still shows excellent performance. Corrosion resistance.

1 Introduction
Plastic parts decorative chrome plating products are widely used in automotive parts due to their aesthetic, wear and corrosion resistance. Chromic acid, which is mostly used in conventional hexavalent chromium plating processes, is very toxic and is a carcinogen. With the strengthening of national environmental protection, the research and application of trivalent chromium plating has received more and more attention. Compared with hexavalent chromium plating, trivalent chromium plating has the following advantages: a. no toxic chromic acid mist is generated during electroplating, and sewage treatment is easy; b. dispersing ability and covering ability of plating solution is better than hexavalent chromium plating process The yield rate is improved; c. The current efficiency of the plating solution is higher than that of the hexavalent chromium plating solution, and the production efficiency is improved without being affected by the current interruption.
This paper is aimed at plating a new type of copper/nickel/trivalent chromium composite coating on the surface of plastic parts provided by an auto parts manufacturer, and accelerating two corrosion samples by acetic acid-salt spray corrosion (CASS) and Russian mud corrosion, combined with electrochemistry. The morphology and corrosion resistance of the new trivalent chromium coating were tested by means of morphological elemental analysis and compared with the traditional hexavalent chromium coating to investigate the corrosion resistance of the trivalent chromium coating. The main factor of sex is to provide a reliable basis for the final replacement of hexavalent chromium.
2, the test part
2.1 Preparation of chrome plating
The surface of the plastic part was plated with Cu (20 μm) + Ni (25 μm) + Cr (0.3 - 0.5 μm). The sulphate trivalent chrome plating process is mainly composed of chromium sulfate, ferrous sulfate, complexing agent, accelerator, boric acid, sodium sulfate, sodium lauryl sulfate, pH 2~3, current density 15- 45A/dm2, temperature 25~45°C. In order to evaluate the quality of the Cr layer, a plastic sample of Cu (20 μm) + Ni (25 μm) + conventional hexavalent chromium plating (0.3 ~ 0.5 μm) was selected for comparison.
2.2 Coating surface morphology and structural composition
X-ray photoelectron spectroscopy (XPS, PHI-5700ES-CA) was used to analyze the chemical state of the sample elements. The chamber vacuum was 6.65x10-8Pa, and the X-ray source was 1C ray of the aluminum target. Scanning electron microscopy (SEM, S- Model 4700) and its accompanying spectrometer (EDS) observe the microscopic morphology of the sample and analyze the content of the main constituent elements of the sample.
2.3 plating corrosion resistance
2.3.1 CASS test
Refer to GB/T10125-2012 "Artificial Atmosphere Corrosion Test Salt Spray Test" for implementation, timing sampling test.
2.3.2 Resistance to Russian mud corrosion test
2.3.2.1 Preparation of Russian mud
a. Configuring a saturated solution of calcium chloride at 40t;
b. Add a saturated calcium chloride solution to an appropriate amount of kaolin (per 5 g of kaolin plus 5 ml of a saturated solution of calcium chloride);
c. Adjust the pH to between 6.5 and 7.5 with 0.05 mol/L sodium hydroxide solution.2.3.2.2 Russian mud corrosion test
A round cake-shaped Russian mud with a diameter of 18 mm (about 0.3 g) was applied to the surface of the sample to be tested, and then the sample was placed at (60 ± 3) t, humidity of 23%, ± 5%, and a high-low temperature humid heat box (BG) /TH Shanghai Bogong Experimental Equipment Factory), timing sampling test.
2.3.3 Electrochemical test
The samples subjected to CASS and Russian mud corrosion tests were tested by electrochemical impedance (EIS) using the P4000 electrochemical integrated test system (EG&G, USA). The test parameters are as follows.
a. The amplitude of the AC signal is 5mV, and the frequency response range is 105~10-1 Hz, and the open circuit potential is measured.
b. The test was carried out at room temperature, the medium was 3.5%, aqueous NaCl solution, a three-electrode system was used, the sample was used as the research electrode, the drill was used as the auxiliary electrode, and the saturated calomel electrode (SCE) was used as the reference electrode.
3 results and discussion
3.1 Analysis of surface elements of the bond layer
Figure 1 shows the surface X-ray photoelectron spectroscopy of the hexavalent chromium process. It can be seen from Fig. 1 that the surface of the hexavalent chromium system is mainly composed of Cr elemental substance, and Fe element is not detected, indicating that there is no other impurity in the surface Cr layer; no Ni element is detected, indicating that X-ray cannot penetrate the Cr layer, and chrome plating The layer is denser.
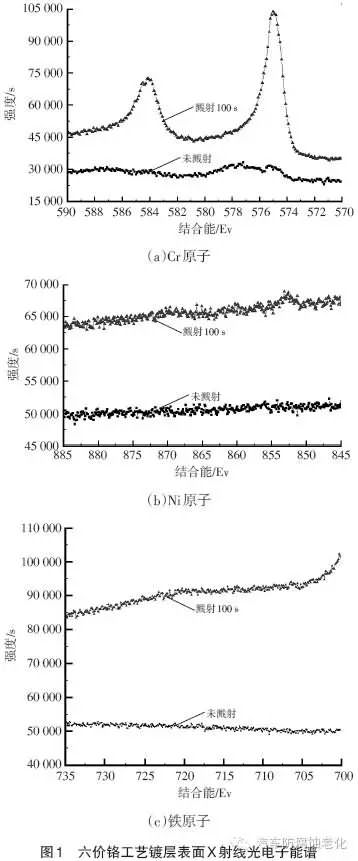
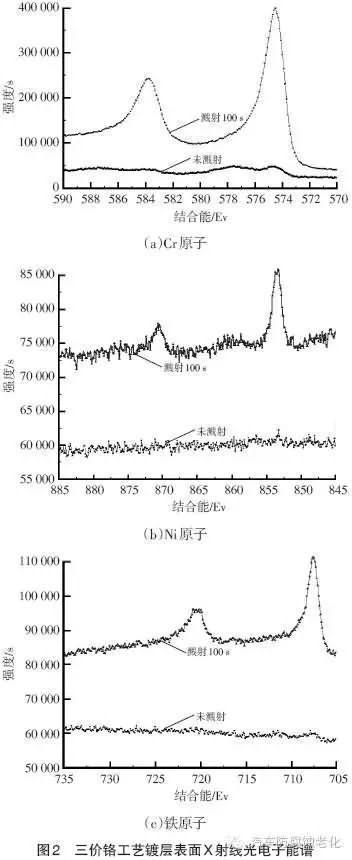
Figure 2 shows the surface X-ray photoelectron spectroscopy of the trivalent chromium process. It can be seen from Fig. 2 that the surface of the trivalent chromium system is mainly composed of Cr elemental substance, and Fe element is detected at the same time. The element is mainly derived from Fe2+ in the chrome plating solution, which is an impurity element in the chrome plating layer; in addition, Ni element is also detected. This is because the X-ray penetrates the Cr layer and reaches the second layer of nickel plating, indicating that the surface of the chrome plating has micropores.
3.2 Electrochemical test results of chrome plating
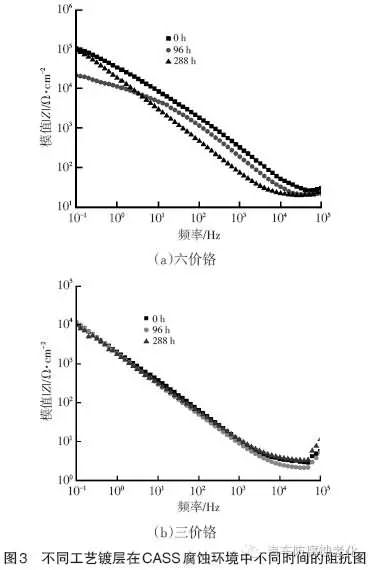
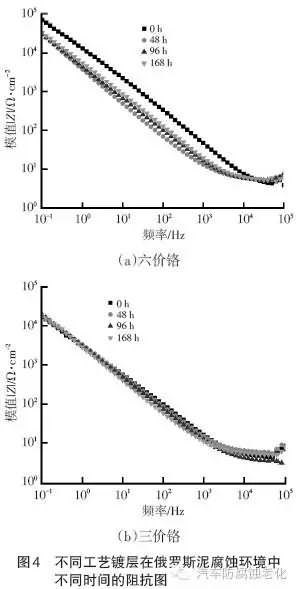
Figure 3 and Figure 4 show the electrochemical impedance spectra of the hexavalent chromium system coating and the trivalent chromium system coating in different corrosion environments of CASS and Russian mud, respectively. The larger the IZI value, the corrosion resistance of the coating. The better. It can be seen from Fig. 3 that with the extension of CASS corrosion time, the IZI value of the hexavalent chromium system coating first decreases from 105a/cm2 to 1.25X104a/cm2, and then returns to 105a/cm2; while the trivalent chromium system is coated. The IZI value is always maintained at 104a/cm2. The above test results show that in the CASS corrosive environment, the hexavalent chromium system coating shows good corrosion resistance and has a certain self-repairing ability; while the corrosion resistance of the trivalent chromium system coating is weak, but after 288h of CASS After the test, it still has stable protection. It can be seen from Fig. 4 that the IZI value of the hexavalent chromium system coating decreases from 1047a/cm2 to 1042a/cm2 with the extension of the Russian mud corrosion time; the IZI value of the trivalent chromium coating is always maintained at 1.25X104Ω/cm2. The above test results show that in the Russian mud corrosion environment, the hexavalent chromium system coating shows good corrosion resistance at the initial stage, and the corrosion resistance gap between the hexavalent chromium and the trivalent chromium system increases with the corrosion time. Gradually shrink.
3.3 Corrosion morphology and corrosion product analysis
Figure 5 is a SEM image of the cadmium chromium system after 520h CASS corrosion test, showing a small amount of corrosion products on the surface of the chrome layer (Zone 1), most of the other areas are still covered by the complete chrome layer (Zone 2) . 6 and 7 are EDS spectra of Region 1 and Region 2, respectively, and Table 1 and Table 2 are the element contents of Region 1 and Region 2. As seen from Fig. 6 and Table 1, the elements of O, Si, Cl, Cr and Ni were detected in the region 1, and the mass percentages were 29.12%, 0.97%, 2.25%, 21.45%, and 46.20%, respectively. Among them, the Cr element and the Ni element are derived from the composite plating layer, the Cl element is derived from the corrosion product, the Si element is derived from the impurity, and the O element may be derived from the oxide or corrosion product of the plating layer. It can be seen from Fig. 7 and Table 2 that the elements detected in the region 2 are O, Cr and Ni, and the mass percentages are 8.39%, 34.74%, and 56.87%, respectively. Among them, the Cr element and the Ni element are derived from the composite plating layer, and the O element may be derived from the oxide of the plating layer.

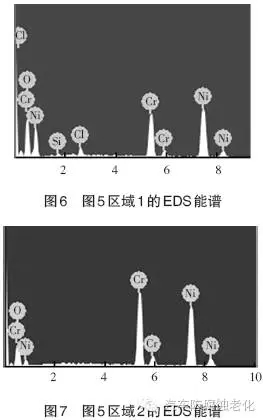
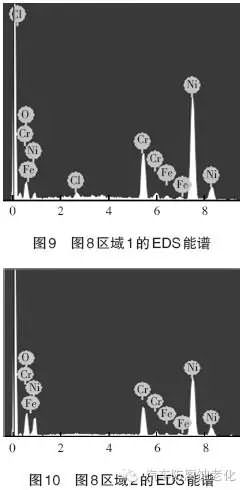
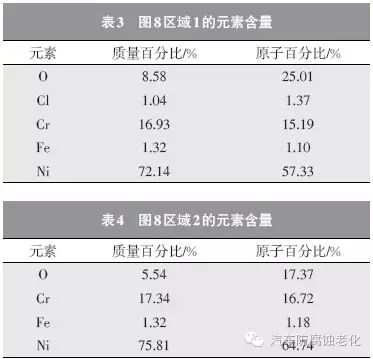
Figure 8 is a SEM image of the microstructure of the trivalent chromium system after 520h CASS corrosion test. It can be seen that the surface of the chrome layer is seriously damaged, and most areas have obvious corrosion (Zone 2). Only part of the chrome layer remains. Complete (Zone 1). 9 and 10 are EDS spectrums of the regions 1 and 2, respectively, and Tables 3 and 4 are the element contents of the regions 1 and 2. As seen from Fig. 9 and Table 3, the elements of O, Cl, Cr, Fe and Ni were detected in the region 1, and the mass percentages were 8.58%, 1.04%, 16.93%, 1.32%, and 72.14%, respectively. The Cr element, the Ni element and the Fe element are derived from the composite plating layer, the Cl element is derived from the corrosion product, and the O element may be derived from the oxide or corrosion product of the plating layer. As seen from Fig. 10 and Table 4, the elements detected in the region 2 have O, Cr, Fe and Ni elements, and the mass percentages are 5.54%, 17.34%, 1.32% and 75.81%, respectively. The Cr element, the Ni element and the Fe element are derived from the composite plating layer, and the O element may be derived from the oxide of the plating layer.
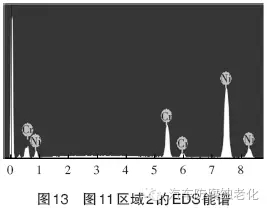
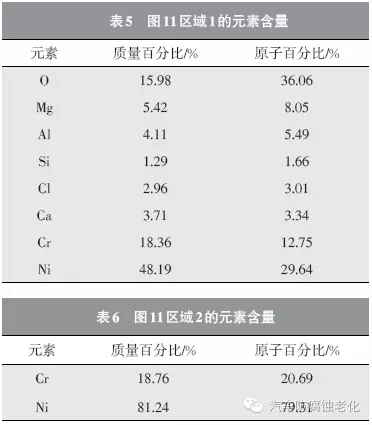
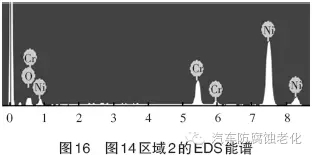
The SEM image of the micro-morphology after the corrosion test shows that the chrome layer has a good surface integrity (Zone 2), and only corrosion occurs in the local area (Zone 1). 12 and 13 are EDS spectra of Region 1 and Region 2, respectively, and Tables 5 and 6 are the element contents of Region 1 and Region 2. As seen from Fig. 12 and Table 5, the regions 1 detected O, Mg, Al, Si, Cl, Ca, Cr and Ni elements, the mass percentages were 15.78%, 5.42%, 4.11%, 1.29%, 2.96%, 3.71, respectively. %, 18.36%, 48.19%,. Among them, Cr element and Ni element are from composite coating, Cl element is from corrosion product, Mg, Al, Si, Ca and other elements are from Russian mud corrosion medium, and O element may come from coating oxide or corrosion product. As seen from Fig. 13 and Table 6, the elements detected in the region 2 are Cr element and Ni element, and the mass percentages are 18.76% and 81.24%, respectively, and the two elements are derived from the composite plating layer.
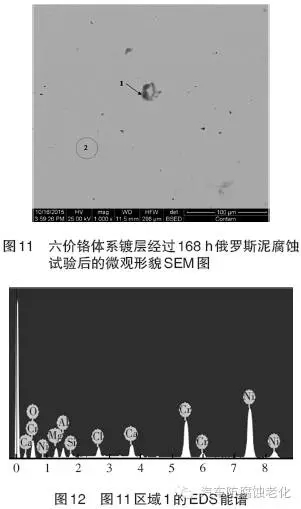
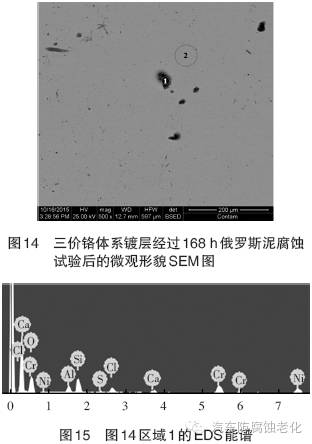
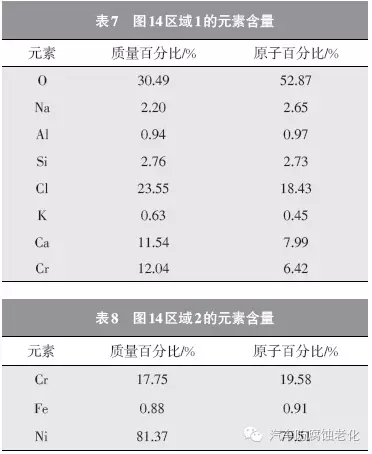
Figure 14 is a SEM image of the microstructure of the trivalent chromium system after 168h Russian mud corrosion test. It can be seen that the chrome layer has a good surface integrity (Zone 2) and only corrosion occurs in the local area (Zone 1). 15 and 16 are EDS spectra of Region 1 and Region 2, respectively, and Tables 7 and 8 are the element contents of Region 1 and Region 2. It can be seen from Fig. 15 and Table 7 that the elements of O, Na, Al, Si, Cl, K, Ca and Cr are detected in the region 1, and the mass percentages are 30.49%, 2.20%, 0.94%, 2.76%, 23.55%, 0.63, respectively. %, 11.54%, 12.04%. Among them, Cr element and Ni element are from composite coating, Cl element is derived from corrosion product, Al, Si, K, Ca and other elements are derived from corrosive medium, and O element may come from oxide or corrosion product of coating. As seen from Fig. 16 and Table 8, the elements detected in the region 2 are Cr element, Fe element and Ni element, and the mass percentages are 17.75%, 0.88%, and 81.37%, respectively, and the three elements are derived from the composite plating layer.
In summary, the hexavalent chromium system coating exhibits good corrosion resistance in both CASS and Russian mud environments. The main reasons are as follows: a. No other impurity elements are found in the chrome plating layer; b. chrome plating surface The structure is dense and can greatly improve the protective life of the coating. In contrast, the corrosion resistance of the trivalent chromium system coating has decreased, but it still has good corrosion resistance in the above two corrosive environments. The main reasons are as follows: a. The chromium coating contains Fe impurity elements, but Due to the low content of Fe, the presence of Fe impurity in the coating will not seriously affect the corrosion resistance of the coating; the Ni element is detected in the trivalent chromium layer, indicating that there are micropores on the surface of the chrome plating, although it is certain To a certain extent, it affects the compactness of the coating of trivalent chromium system, but the corrosion resistance of the coating will not be greatly reduced in a certain period of time.4 Conclusion
a. Compared with the traditional hexavalent chromium system coating, the iron element and the plating density in the trivalent chromium system coating are the main factors causing the corrosion resistance of the coating to decrease slightly, but the new trivalent chromium system coating still shows excellent resistance. Corrosion performance
b. The Ni element in the trivalent chromium layer indicates that the surface of the trivalent chromium plating layer has micropores locally, but the corrosion resistance of the plating layer does not greatly decrease in a certain period of time;
c. From the perspective of environmental protection and coating performance, the new trivalent chromium plating process involved in this paper can replace the traditional hexavalent chromium plating process.
Source: Automotive Technology and Materials, 2016, third issue.Zhang Jing Guo Xinghua Du Keqin Guo Quanzhong Gao Chengyong
(1. Technology Center of China First Automobile Co., Ltd., Changchun 130011;
2. Laboratory of Corrosion and Protection, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110000, China)
Metal Shell LED Downlight Home Office Supermarket Lighting
home office super market hospital ceiling light lighting
Metal Shell Led Downlight,Home Office Led Downlight,Supermarket Led Downlight,Lighting Led Downlight
Jiangmen hengshenghui Lighting Co., Ltd , https://www.jmsunbright.com