Metal lithium anodes with high theoretical specific capacity and low oxidation-reduction potential are expected to help realize the next generation of high-energy batteries. However, the dendrite problem of metal lithium anodes in liquid electrolyte systems has been criticized. Dendrite growth can not only lead to irreversible capacity loss of lithium, but also may cause battery short circuit and even explosion. Scientists have conducted extensive research on the growth mechanism of dendrites. Among them, the widely recognized Chazalviel model points out that the dendrite nucleation time is affected by the electrolyte ion concentration, anion / cation mobility and effective current density. Increasing the lithium ion mobility of the electrolyte and reducing the anion mobility will effectively extend the nucleation time and inhibit dendrite growth.
Recently, the Suzhou Institute of Nanotechnology and Nanobionics, Chinese Academy of Sciences, has designed an asymmetric gel polymer electrolyte (Asymmetric GPE) membrane that promotes rapid lithium ion transport and uniform deposition for dendrite-free, based on the mechanism of dendrite growth Growing metal lithium battery.
First, the molecular dynamics (MD) simulation results prove that the polar units on the PVDF molecular chain can bind PF6- in the electrolyte with ion-dipole force (Figure 1c), and Li + shows higher in the system The diffusion coefficient (Figure 1e). Using this property of the polymer, the team designed a unique membrane structure to adjust the electrolyte ion distribution. Among them, the vertical channel layer that occupies the main part can shorten the internal transmission path and realize the rapid conduction of ions; the nanopore layer on the contact surface with the lithium negative electrode plays the role of redistributing and homogenizing the flow of lithium ions. During the charging process, PF6- is bound to the polymer matrix, and Li + can be quickly conducted to the negative electrode and deposited uniformly, thereby achieving a dendritic metal lithium battery.
The polymer membrane of this asymmetric structure is produced by freeze casting combined with phase inversion (Figure 2a). The DMSO solution of PVDF-HFP was scraped on a copper plate and transferred to a low-temperature copper cooler, using the nucleation and directional growth of solvent ice crystals under a temperature gradient to form a unique asymmetric structure, and solidified in a non-solvent. According to SEM characterization, the main part of the membrane has a parallel arrangement of pores, while the bottom layer is a relatively dense nanopore structure, and the upper and lower surfaces are correspondingly porous and dense. This asymmetric PVDF-HFP membrane is activated with electrolyte (1 M LiPF6 in EC / DEC) to obtain Asymmetric GPE. Compared with Porous GPE based on porous membrane, Asymmetric GPE has higher porosity, electrolyte adsorption rate, lower internal tortuosity, and better mechanical properties.
After testing, Asymmetric GPE has a high lithium ion migration number t + (0.66) that is consistent with the calculated results, which is significantly better than the liquid electrolyte (0.34). At the same time, Asymmetric GPE with unique channel structure exhibits excellent ion transmission performance. At 20 ℃, its ionic conductivity is 3.36 mS cm-1, which is close to pure liquid electrolyte, which is better than Porous GPE and commercial Celgard membrane. The excellent lithium ion conductivity of Asymmetric GPE will help the realization of non-dendritic lithium anodes and high-performance lithium metal batteries.
The researchers characterized the deposition morphology of lithium metal. The lithium deposition layer under Asymmetric GPE is dense and smooth, while there are many uneven lithium dendrites under the liquid electrolyte (Figure 5a-d). At the same time, the GPE deposition process has a lower nucleation and steady-state potential, which means excellent deposition kinetics (Figure 5e). Li | Li symmetrical batteries also confirmed this conclusion (Figure 5f). Under the conditions of 1 mA cm-2 and 1 mAh cm-2, the GPE battery has a lower cycle overpotential and a stable cycle of more than 250 hours; while the liquid battery begins to become unstable after 60 hours of cycling and fails after 200 hours. These results demonstrate the effective inhibition of lithium dendrite growth by Asymmetric GPE.
The researchers assembled lithium iron phosphate (LFP) as a positive metal lithium battery, further verifying the excellent performance of Asymmetric GPE. First, the GPE battery has a lower interface impedance (Figure 6a), indicating a tighter fit of the electrode and electrolyte and a more uniform ion distribution. At 30 ° C, GPE batteries exhibit a high discharge specific capacity of 156 mAh g-1 at 0.2 C rate, and also have specific capacities of 149, 140 and 101 mAh g-1 at 0.5 C, 1 C and 5 C, far Liquid batteries with higher than the same amount of electrolyte (Figure 6b). In the 2 C cycle test, the average coulombic efficiency reached 99.5% after the GPE battery circulated steadily for 600 cycles, while the capacity of the liquid battery decayed rapidly after 300 cycles and the average coulombic efficiency was only 97.9% (Figure 6c). The results of the LFP | Li battery indicate that Asymmetric GPE with fast lithium ion conduction and suppression of lithium dendrites helps to maintain high coulombic efficiency and stable cycling of lithium metal batteries.
The above research results were published in Journal of Materials Chemistry A (doi.org/10.1039/D0TA01883J) under the title of Asymmetric Gel Polymer Electrolyte with High Lithium Ionic Conductivity for Dendrite-free Lithium Metal Batteries. The first author is Li Linge, a graduate student of the Chinese University of Science and Technology, and the corresponding author is project researcher Liu Meinan.

Figure 1. (a) Asymmetric GPE and (b) ion transmission diagram. Calculate the radial distribution functions of (c) PF6- and (d) Li + and PVDF, (e) the diffusion coefficient of ions in PVDF.
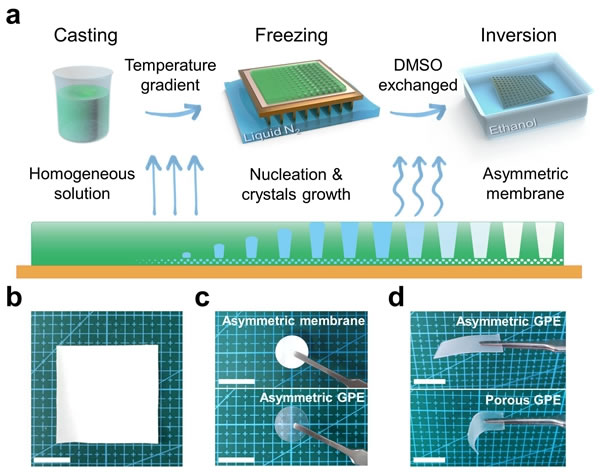
Figure 2. (a) Schematic diagram of asymmetric polymer membrane preparation. (Bd) Physical picture of asymmetric polymer membrane GPE.
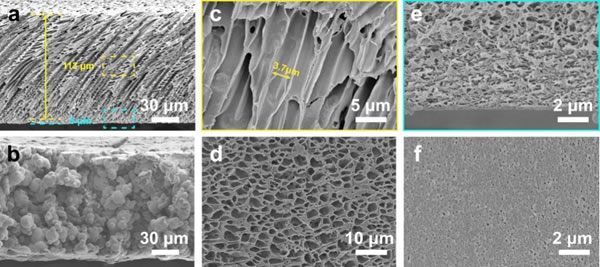
Figure 3. SEM image of polymer film. (A) Comparative cross-section of asymmetric polymer membrane and (b) porous membrane, (c, d) cross-section and surface of parallel channel layer and (e, f) nanopore layer.

Figure 4. Characterization of electrolyte ion transport performance. (A) Asymmetric GPE lithium ion migration number test, (b) Raman spectroscopy, (c) ion conductivity Arrhenius curve and (d) ion transmission performance.
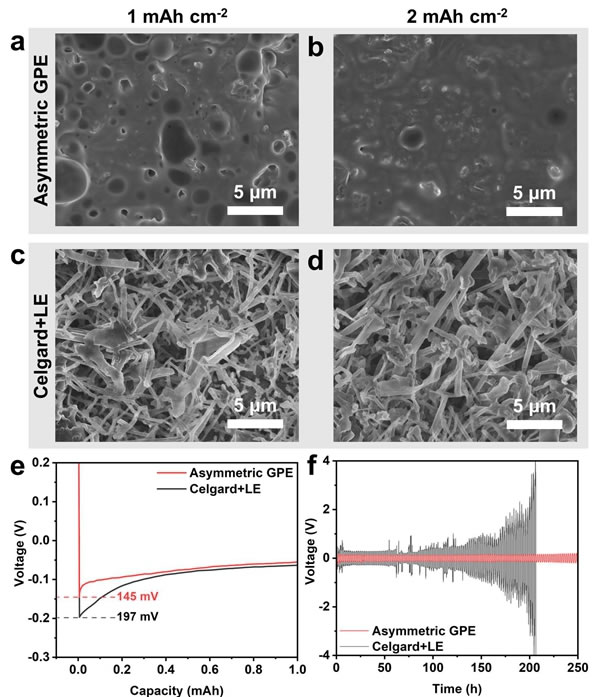
Figure 5. Effect of electrolyte on the deposition of metallic lithium. (Ad) Asymmetric GPE and Celgard + LE deposit SEM image of lithium morphology on copper foil surface, (e) deposition potential of metallic lithium, (f) cyclic overpotential of Li | Li symmetric battery
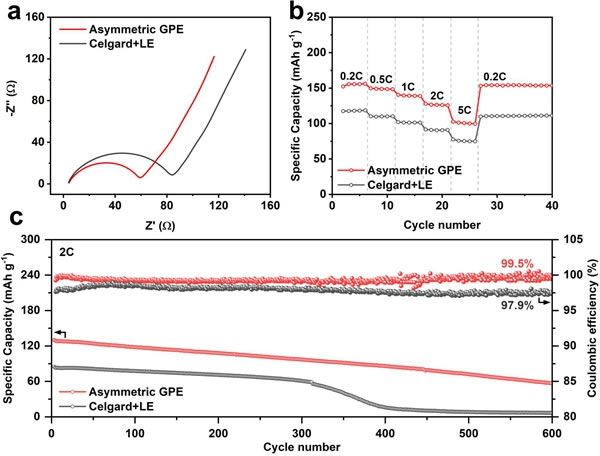
Figure 6. Li | LFP battery test. Asymmetric GPE and Celgard + LE (a) battery impedance test, (b) rate performance, (c) long cycle performance and Coulomb efficiency.
soap rack,soap holder,soap stand,stainless steel soap holder,steel wire soap stand,etc.Let your bathroom become more simple and upscale!Applicable to families, hotels, home stay and other places to use.
304 stainless steel never rust, will easy to clear, it's also very durable!
we are 15 year factory, we had big engineer team, and strong production line, can give you good serve and quanlity. Welcome to cooperation!
Soap Rack,Soap Holder,Soap Stand,Stainless Steel Soap Holder,Stainless Steel Wire Soap Stand
Shenzhen Lanejoy Technology Co.,LTD , https://www.wirefruitbasket.com